Promising information from giant Alzheimer’s research bodes properly for this Seattle startup

Alzheimer’s disease researchers were buoyed by positive data released Tuesday from a large clinical trial of an experimental therapy being developed by Biogen and Eisai.
The early data suggest that the treatment, called lecanemab, eases cognitive decline. The findings also rejuvenated hope in a therapeutic approach targeting peptides that form nasty clumps in the brain of people with Alzheimer’s.
The news bodes well for companies like Seattle’s AltPep that are taking a similar path toward detecting and treating the disease.
“We see the positive results reported for lecanemab as supportive of our approach,” said AltPep founder and CEO Valerie Daggett.
Biopharma companies have spent billions of dollars over decades on failed trials for Alzheimer’s directed at the target, amyloid-beta. The hypothesis behind the trials is that the clumps of amyloid-beta cause the symptoms of disease, instead of being protective or neutral.
That same approach lies behind Biogen’s controversial drug Aduhelm (aducanumab), approved by the U.S. Food and Drug Administration last year on limited data. Aduhelm received coverage from Medicare only in the context of clinical trials and has not been commercially successful.
The new findings support the view that approaches targeting amyloid-beta may, in the end, be effective.
Lecanemab also targets amyloid-beta, but appears to have a more pronounced effect than Aduhelm and has fewer side effects. The new therapy reduced clinical decline by 27% at 18 months compared to a placebo in the trial of 1,800 people with mild cognitive impairment or mild Alzheimer’s. The phase 3 findings were provided a press release and generated a mixed reception from experts; more complete data is expected in late November.
“They have not yet made the full data set publicly available, but from what they have provided, it is very encouraging that they see a positive effect on cognition and lifestyle metrics,” said Daggett, who is also a University of Washington bioengineering professor.
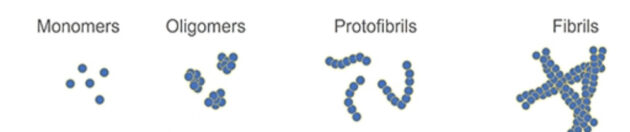
Amyloid-beta is found in several different forms, and the new therapy may be hitting the right component, said Daggett. Amyloid-beta congregates into protofibrils and fibrils before finally forming clumps called plaques.
Lecanemab was developed against protofibrils but it also binds to other forms including one found early in disease called an oligomer. Such oligomers are thought thought to be toxic and trigger a host of Alzheimer’s pathologies. And Lecanemab probably works against these toxic oligomers, said Daggett.
“We know that past approaches targeting amyloid plaques, fibrils and protofibrils have not been effective,” said Daggett. “These new results support a recasting of the amyloid hypothesis with emphasis on targeting the amyloid-beta toxic oligomers form, which begin to form 10-20 years before clinical diagnosis of mild cognitive impairment.”
AtlPep is similarly developing agents that target the toxic oligomers. “We’re going after that first molecular trigger for the pathology,” Daggett previously told GeekWire.
AltPep is developing a diagnostic test for Alzheimer’s disease and testing its agents as potential therapies in preclinical studies. The startup recently raised $44.4 million to propel its efforts.
And while lecanemab is a monoclonal antibody administered via intravenous infusion, AltPep’s peptide agents are being developed as easy-to-administer intranasal therapies.
Some researchers say that Alzheimer’s may ultimately yield to a combination of approaches, as other events in the brain are also thought to contribute to the disease.
Another Seattle biotech company, Athira Pharma, is taking a distinct approach to the condition. Athira’s experimental compound fosgonimeton is designed to affect the activity of molecules involved in the growth of neurons. The compound, however, has proven disappointing so far in ongoing phase 2 trials. Athira’s stock value plummeted in June after the release of interim data.
Most of the patients in Athira’s study received fosgonimeton in combination with existing Alzheimer’s drugs. But there was a glimmer of hope in the data on people who took only Athira’s compound: the findings hinted that it might have a potential benefit as a monotherapy. Athira has since adapted its study to focus on evaluating patients receiving only fosgonimeton.
Meanwhile, the FDA is expected to make a decision in January on whether to grant accelerated approval to lecanemab. Roche and Eli Lilly are also developing amyloid-beta targeting therapies; data on two Roche studies are expected by the end of the year and the FDA is on deck to rule on accelerated approval for the Eli Lilly candidate in January.
Conclusion: So above is the Promising information from giant Alzheimer’s research bodes properly for this Seattle startup article. Hopefully with this article you can help you in life, always follow and read our good articles on the website: Doshared.com




